Results from clinical trials of risdiplam (RG7916;
Genentech, South San Francisco, CA) for treatment of infants with spinal muscle
atrophy (SMA) were presented at the World Muscle Society Congress in Mendoza, Argentina.
In the interim clinical trial data presented, patients with
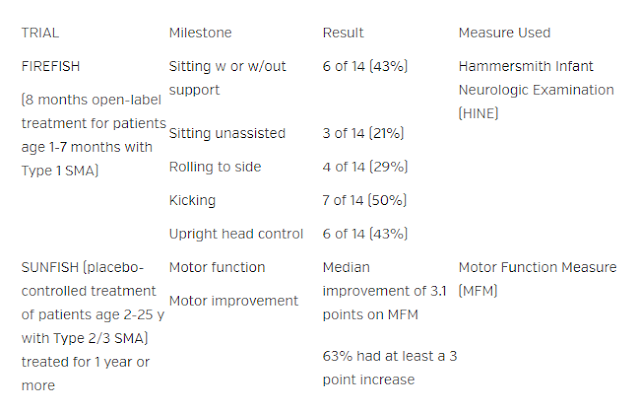
Type 1 SMA who were treated with risdiplam in the open-label study FIREFISH
(NCT ) met motor milestones after 8 months of treatment (Table). In the
double-blind placebo-controlled SUNFISH (NCT )study, patients with Type 2/3 SMA
treated with risdiplam for 1 year or more had improvements in motor function.
No patients withdrew from any of the 3 trials underway because of drug-related
safety concerns.
Of the 20 patients enrolled in the FIREFISH study, 19 have
survived and 3 have reached age 24 months. Since starting treatment, none of
the 19 still-enrolled patients have needed tracheostomy or ventilatory support
or lost the ability to swallow.
“We are highly
encouraged by these data showing infants treated with risdiplam surviving and
achieving developmental milestones beyond the natural history of this
devastating disease,” said Sandra Horning, MD, chief medical officer and head
of Global Product Development. “SMA therapies that produce a sustained increase
in SMN protein in both the CNS and periphery may provide comprehensive benefits
to people diagnosed with SMA, and we look forward to sharing additional data on
risdiplam as the clinical program progresses.”
Although SMA is a rare disease, it is one of the most common
genetic diseases, affecting approximately 1 in 11,000 babies. Until the first
treatment, nusinersen (Spinraza, Biogen; Cambridge, MA) became available in
late 2016, SMA was considered a fatal disease with the majority of
patients—those with Type 1 SMA—not surviving past early childhood. Patients
born with Type 2 or Type 3 SMA have longer life expectancies, although with
significant disability including loss of physical strength and ability to walk,
eat, or breathe significantly diminished.
http://practicalneurology.com/NEWS/?ID=52658&CENTER=181&utm_campaign=Neurologywire&utm_source=hs_email&utm_medium=email&utm_content=66444634&_hsenc=p2ANqtz-8R8xQjoqpxcvJMng6-uEKASowsppn3l4R3e5G4n5bXCkQJiQ04JPFZfgNEdd-Hngbm8LGzK5zv3Q_QBaKAhEg6pzZkkjISpifdHJxq58k282gz5pM&_hsmi=66444634
No comments:
Post a Comment