Nguyen TTM, Murakami Y, Wigby KM, Baratang NV, Rousseau J, St-Denis A, Rosenfeld JA, Laniewski SC, Jones J, Iglesias AD, Jones MC, Masser-Frye D, Scheuerle AE, Perry DL, Taft RJ, Le Deist F, Thompson M, Kinoshita T, Campeau PM. Mutations in PIGS, Encoding a GPI Transamidase, Cause a Neurological Syndrome Ranging from Fetal Akinesia to Epileptic Encephalopathy. Am J Hum Genet. 2018 Oct 4;103(4):602-611.
Abstract
Inherited GPI deficiencies (IGDs) are a subset of congenital
disorders of glycosylation that are increasingly recognized as a result of
advances in whole-exome sequencing (WES) and whole-genome sequencing (WGS).
IGDs cause a series of overlapping phenotypes consisting of seizures,
dysmorphic features, multiple congenital malformations, and severe intellectual
disability. We present a study of six individuals from three unrelated families
in which WES or WGS identified bi-allelic phosphatidylinositol glycan class S
(PIGS) biosynthesis mutations. Phenotypes included severe global developmental
delay, seizures (partly responding to pyridoxine), hypotonia, weakness, ataxia,
and dysmorphic facial features. Two of them had compound-heterozygous variants
c.108G>A (p.Trp36∗) and c.101T>C (p.Leu34Pro), and two siblings of
another family were homozygous for a deletion and insertion leading to
p.Thr439_Lys451delinsArgLeuLeu. The third family had two fetuses with multiple
joint contractures consistent with fetal akinesia. They were compound
heterozygous for c.923A>G (p.Glu308Gly) and c.468+1G>C, a splicing
mutation. Flow-cytometry analyses demonstrated that the individuals with PIGS
mutations show a GPI-AP deficiency profile. Expression of the p.Trp36∗
variant in PIGS-deficient HEK293 cells revealed only partial restoration of
cell-surface GPI-APs. In terms of both biochemistry and phenotype, loss of
function of PIGS shares features with PIGT deficiency and other IGDs. This
study contributes to the understanding of the GPI-AP biosynthesis pathway by
describing the consequences of PIGS disruption in humans and extending the
family of IGDs.
Yang L, Peng J, Yin XM, Pang N, Chen C, Wu TH, Zou XM, Yin
F. Homozygous PIGT Mutation Lead to Multiple Congenital Anomalies-Hypotonia
Seizures Syndrome 3. Front Genet. 2018 May 8;9:153.
PIGT encodes a subunit of the glycosylphosphatidylinositol
transamidase complex, which catalyzes the attachment of proteins to
GPI-anchors. A homozygous PIGT variant c.550G>A (p. E184K) in a Chinese boy
with multiple malformations, hypotonia, seizure and profound development delay
was identified by panel sequencing. Pathogenicity of the variant was confirmed
by flow cytometry. The expression of CD16 and CD24 of this proband reduced to
16.92 and 22.16% compare with normal control respectively while which of his
parents and sister were normal. This mutation raised the mRNA level on the
peripheral blood mono nuclear cells of this patient. This study expanded the
variant spectrum of MCAHS3, and CD16 could be an effective marker to evaluate
the pathogenicity of PIGT mutation.
Nguyen TTM, Murakami Y, Sheridan E, Ehresmann S, Rousseau J,
St-Denis A, Chai G, Ajeawung NF, Fairbrother L, Reimschisel T, Bateman A,
Berry-Kravis E, Xia F, Tardif J, Parry DA, Logan CV, Diggle C, Bennett CP, Hattingh
L, Rosenfeld JA, Perry MS, Parker MJ, Le Deist F, Zaki MS, Ignatius E,
Isohanni P, Lönnqvist T, Carroll CJ, Johnson CA, Gleeson JG, Kinoshita T, Campeau PM.
Mutations in GPAA1, Encoding a GPI Transamidase Complex Protein, Cause
Developmental Delay, Epilepsy, Cerebellar Atrophy, and Osteopenia. Am J Hum Genet. 2017 Nov
2;101(5):856-865.
Abstract
Approximately one in every 200 mammalian proteins is
anchored to the cell membrane through a glycosylphosphatidylinositol (GPI)
anchor. These proteins play important roles notably in neurological development
and function. To date, more than 20 genes have been implicated in the
biogenesis of GPI-anchored proteins. GPAA1 (glycosylphosphatidylinositol anchor
attachment 1) is an essential component of the transamidase complex along with
PIGK, PIGS, PIGT, and PIGU (phosphatidylinositol-glycan biosynthesis classes K,
S, T, and U, respectively). This complex orchestrates the attachment of the GPI
anchor to the C terminus of precursor proteins in the endoplasmic reticulum.
Here, we report bi-allelic mutations in GPAA1 in ten individuals from five
families. Using whole-exome sequencing, we identified two frameshift mutations
(c.981_993del [p.Gln327Hisfs∗102] and c.920delG [p.Gly307Alafs∗11]),
one intronic splicing mutation (c.1164+5C>T), and six missense mutations
(c.152C>T [p.Ser51Leu], c.160_161delinsAA [p.Ala54Asn], c.527G>C
[p.Trp176Ser], c.869T>C [p.Leu290Pro], c.872T>C [p.Leu291Pro], and
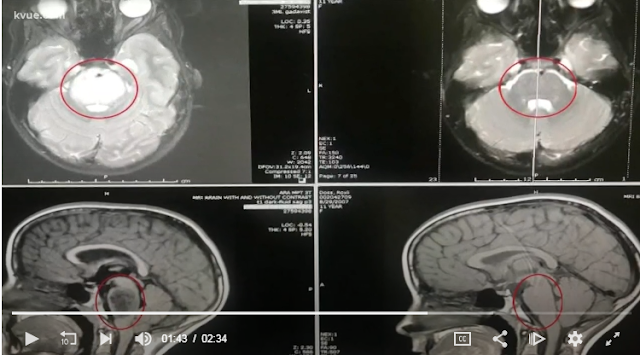
c.1165G>C [p.Ala389Pro]). Most individuals presented with global
developmental delay, hypotonia, early-onset seizures, cerebellar atrophy, and
osteopenia. The splicing mutation was found to decrease GPAA1 mRNA. Moreover,
flow-cytometry analysis of five available individual samples showed that
several GPI-anchored proteins had decreased cell-surface abundance in
leukocytes (FLAER, CD16, and CD59) or fibroblasts (CD73 and CD109).
Transduction of fibroblasts with a lentivirus encoding the wild-type protein
partially rescued the deficiency of GPI-anchored proteins. These findings
highlight the role of the transamidase complex in the development and function
of the cerebellum and the skeletal system.