Coughlin , C.R., II and Gospe , S.M., Jr. (2023), Pyridoxine-dependent epilepsy: Current perspectives and questions for future research. Ann Child Neurol Soc, 1: 24-37. https://doi.org/10.1002/cns3.20016
Abstract
Pyridoxine-dependent epilepsy (PDE) was historically defined by a dramatic clinical response to a trial of pyridoxine and the re-emergence of seizures after withdrawal of pyridoxine. Research conducted over the last seven decades has revealed that the phenotype of PDE results from multiple genetic disorders, and the most common disorder, PDE-ALDH7A1, is caused by a deficiency of an enzyme involved in lysine metabolism. PDE-ALDH7A1 is characterized by more than epilepsy, as many patients have abnormalities of brain development, and most patients have intellectual and developmental disability. Treatment aimed at the underlying metabolic defect, in addition to pyridoxine supplementation, has improved clinical outcomes. Recently discovered biomarkers and genetic testing allow for the diagnosis of PDE-ALDH7A1 without the need of a pyridoxine trial and hold the promise for newborn screening. Despite these many advances, PDE-ALDH7A1 remains a clinical and biochemical conundrum. The increasing use of model systems and an international collaboration of clinician-scientists are among the reasons to be optimistic that these questions will be answered in the near future and that the clinical outcomes and quality of life will continue to improve for patients with PDE-ALDH7A1.
From the article:
Patients with PDE-ALDH7A1 may have congenital and/or progressive abnormalities of brain development. Thinning of the posterior corpus callosum, primarily the isthmus, is universal and can be quantitatively demonstrated via magnetic resonance imaging (MRI) geometric morphometry. In addition, mega cisterna magna and other posterior fossa abnormalities have been noted in approximately 20% of patients. Uncommonly, heterotopia and other forms of cortical dysplasia have been demonstrated with imaging and neuropathological studies. Obstructive hydrocephalus and progressive ventriculomegaly (hydrocephalus ex vacuo, presumably due to late diagnosis and/or poor seizure control) have been described. Other developmental abnormalities include congenital cataracts as well as variable pattern of facial dysmorphism that includes hypertelorism, depressed nasal bridge, epicanthal folds, high hairline, pointed chin, full eyebrows, and broad nasal root (Gospe, unpublished observations)...
Pyridoxine supplementation
PDE is historically defined based on the resolution of seizures following treatment with pharmacologic doses of pyridoxine, and treatment with pyridoxine remains central to the treatment of the epilepsy that has defined this disorder. The importance of vitamin B6 supplementation is highlighted by the recurrence of seizures following pyridoxine withdrawal and case reports that describe patients who died before pyridoxine was administered. As noted above, patients with PDE-ALDH7A1 have a secondary deficiency of PLP most likely due to a Knoevenagel reaction between the accumulating Δ1-P6C and PLP. Treatment with pyridoxine, as opposed to PLP, is recommended for several reasons. There is an association between PLP supplementation and cirrhosis, including at doses usually required for successful treatment of seizures. It is also worth considering that commercial pyridoxine preparations are relatively easy to obtain and stable, whereas PLP degrades quickly when exposed to light. The International PDE Consortium consensus guideline for pyridoxine supplementation by age is listed in Table 2. Note that these pharmacologic doses are substantially higher than the recommended dietary allowance (physiologic doses), which ranges from 0.1 mg for infants to 2 mg for lactating females. While there is a risk of peripheral neuropathy when the dose of pyridoxine exceeds 500 mg/day, the vast majority of patients with PDE-ALDH7A1 do not require such large doses for seizure control. Some patients may require one or more anti-seizure medications, in addition to the maximum recommended dose of pyridoxine, to achieve control of their seizures.
There have been many reports of presumed intrauterine fetal seizures in newborns subsequently diagnosed with PDE-ALDH7A1. As there is a 25% recurrence risk for a couple having another affected child, maternal pyridoxine supplementation during gestation has been proposed for these at-risk pregnancies, specifically to help control intrauterine seizures and improve developmental outcome. Ideally, plans should be made for the fetus to be delivered at an institution that is equipped to care for a newborn at risk of developing an epileptic encephalopathy, and the baby should receive pyridoxine supplementation until the results of confirmatory biochemical studies and/or gene testing have been obtained...
Lysine-reduction therapies
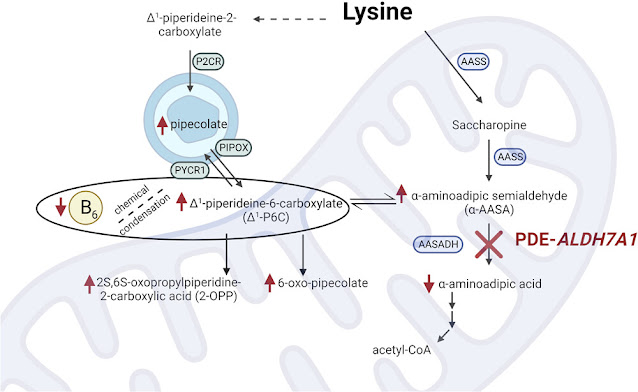
From the early description of the disorder, patients with PDE were described as having intellectual or developmental disability (IDD). More recent studies noted that there was no association between the time of seizure onset or severity and IDD, suggesting the poor developmental outcomes may be due to a separate disease mechanism. The discovery that PDE-ALDH7A1 was, in fact, an organic aciduria led to the current hypothesis that accumulating α-AASA (or related) metabolites are neurotoxic and contribute to the IDD phenotype. Current adjunct (to pyridoxine) therapies attempt to reduce the nutritional intake of lysine or reduce the transport of lysine and are collectively referred to as lysine-reduction therapies (LRTs).
In a cross-sectional study of seven subjects, van Karnebeek et al. treated patients with PDE-ALDH7A1 with pyridoxine and a lysine-free medical formula and demonstrated a decrease in pipecolic acid, α-AASA, and Δ1-P6C. Shortly thereafter, a single patient was treated with pyridoxine and arginine, as the dibasic amino acids lysine, arginine, and ornithine are transported by the same cationic transporter in the intestine, the kidney, and the blood-brain barrier. Following daily arginine supplementation, the patient had a decrease in urine and cerebrospinal fluid (CSF) α-AASA and improved neuropsychiatric testing. In a pre–post design, patients who were initially treated with pyridoxine and lysine-restricted diet had arginine supplementation added to their treatment regimen (also referred to as triple therapy). These patients demonstrated a significant decrease in plasma α-AASA and Δ1-P6C.
Although not the primary outcome measure in these studies, all of the reports noted improved development in those patients treated with a form of LRTs. A recent cohort study evaluated the association between treatment with LRTs and cognitive outcomes. Cohort assignment was based on treatment at the time of developmental testing, and treatment with pyridoxine and LRT was associated with a nonsignificant increase on developmental testing compared to treatment with pyridoxine alone. Notably, treatment with pyridoxine and LRTs in the first six months of life was associated with a significant increase on developmental testing scores, which emphasizes both the efficacy of LRTs and the importance of early, if not newborn, diagnosis and treatment.

No comments:
Post a Comment